Electrochemical Solutions
Introduction
Corrosion of steel reinforcement is both an electrical and a chemical process, which is greatly enhanced when the concrete is under attack from chlorides.
This process involves negatively charged areas of steel being formed called anodes.
It is this point that the corrosion occurs while the remaining positively charged areas of steel (cathodes) remain free from corrosion. By reversing the flow of ions between the two points the same chemical reaction that encourages the corrosion to occur can be used to stop further degradation.
There are several different methods, a very brief overview of each is detailed below.



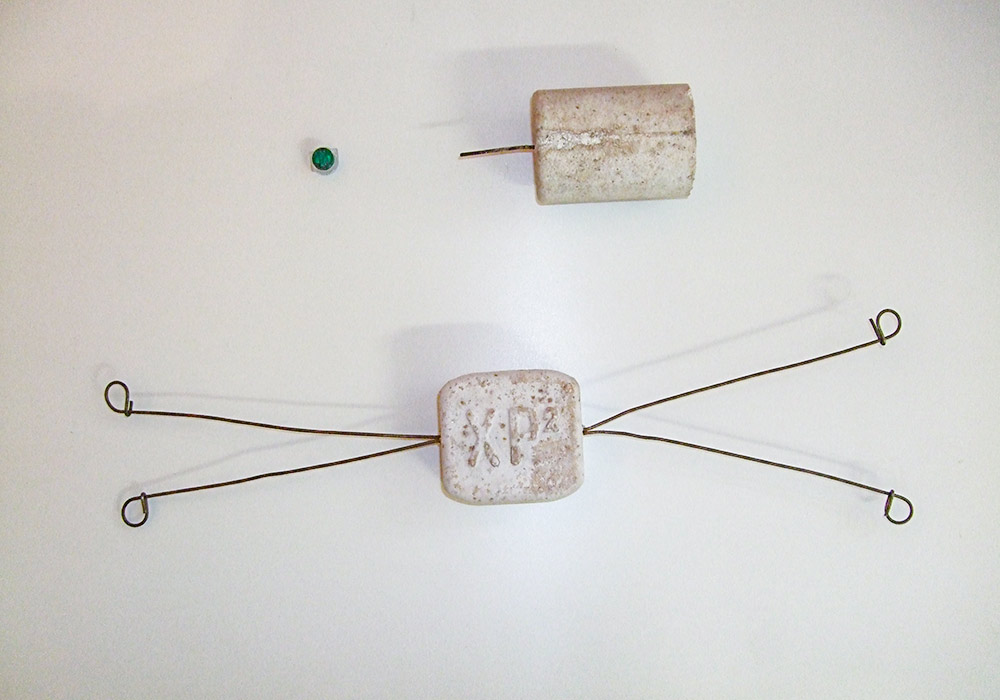
Sacrificial Anodes , Galvanic Systems
Installation of sacrificial anodes either tied direct to the reinforcement surrounding the patch repair or installed within the surrounding concrete (drilled and fixed within the concrete) or fixed onto the surface.
Permanent Impressed Current Installation
This system uses a power supply sending a small electric current between the steel rebar and the anode. A reference electrode is also embedded in the concrete and connected to a computer monitoring system
Temporary Impressed Current Installation
Short term high powered cathodic protection designed to rapidly rehabilitate the concrete cover to steel. Usually activated titanium mesh or similar to concrete surface within an electrolyte reservoir connected to the reinforcement. An electric current induces a migration in and out of electrolyte.
Chloride Extraction -Desalinisation
temporary anode to apply 50v direct to steel reinforcement positive charge repels the negative charged ions rebuilding the passivating layer to steel over 4-6 weeks.
Re-Alkalization
Used for carbonated concrete. The hydroxyl ions at the cathode re-alkalise the concrete from the reinforcement outwards. This process involves a wet anode on the surface containing calcium carbonate, which moves under electro-osmotic pressure , re-alkalises from the surface inwards.
MCI Migrating corrosion Inhibitors
Impregnating chemical solution that when applied to the surface migrates through the concrete to the steel and reforms the passivating layer to the steel.





